Bibliography Background About KRIS
Stream Temperature
California chinook salmon, coho salmon, steelhead trout and coastal cutthroat trout are all Pacific salmon species (genus Oncorhynchus), and all require cold water. Water temperature tolerance varies somewhat between species and also between life stages. Warm temperatures can reduce fecundity, decrease egg survival, retard growth of fry and smolts, reduce rearing densities, increase susceptibility to disease, decrease the ability of young salmon and trout to compete with other species for food and to avoid predation (Spence et al., 1996; McCullough, 1999). See the Amphibian Background page to learn about other cold-water dependent species.
Much of the data on salmonid temperature tolerance is derived from laboratory experiments that may not reflect survival in streams. Lab experiments expose juvenile fish to varying acclimation temperatures, then raise the water temperature at different rates until 50% of the fish die. These tests have established lethal values for coho known as critical thermal maxima (CTM) and upper incipient lethal temperatures (UILT). It has not been established how these values relate to fish stress and mortality in nature. Fish in the wild must forage for food and avoid predation, while in laboratory environments the fish are fed and protected from predators. Stress may occur at lower temperatures in the wild as the fish must cope with all the variables of its environment. For a full discussion of thermal stress terminology see McCullough (1999) p. 10.
Sensitivity by Life Stage
 Eggs
Eggs
Eggs are the most temperature sensitive salmonid life history stage (Hicks 2000). Reiser and Bjornn (1979) defined optimum temperatures for salmon and steelhead egg incubation as 4.4-14.4o C. Hicks (2000) found that "to fully support the pre-emergent stages of coho development that the 7-day average of the daily maximum temperatures should not exceed 9-12° C." Because of the timing of entry by spawners to the streams and moderate coastal climates, exposure of salmon and steelhead eggs to high water temperatures is not likely to be limiting in coastal streams, but can be a factor in interior basins where spring, summer and early fall spawning occur.
Juveniles
 Juvenile coho salmon and steelhead are susceptible to problems related to increased stream temperature because they rear in freshwater over the hot summer and fall months. Steelhead are more tolerant of warmer water temperatures than coho salmon (Frissell, 1992), but may stay in freshwater up to three years, increasing the duration of period subject to temperature problems. Field studies in southwest Oregon streams found that coho, cutthroat and yearling steelhead rearing densities decreased linearly as temperatures exceeded 17° C (Frissell,1992). They also found that coho salmon juveniles were absent in waters that reached 21 - 23° C, except where thermal refugia were available. Juvenile salmonids will not persist in streams where temperature stress exceeds some threshold that can be defined by species and duration of high temperatures. In northern California, both Welsh et al. (2001) and Hines and Ambrose (1998) found that coho salmon juveniles did not persist where the floating weekly maximum temperature exceeded 18.3° C for any length of time.
Juvenile coho salmon and steelhead are susceptible to problems related to increased stream temperature because they rear in freshwater over the hot summer and fall months. Steelhead are more tolerant of warmer water temperatures than coho salmon (Frissell, 1992), but may stay in freshwater up to three years, increasing the duration of period subject to temperature problems. Field studies in southwest Oregon streams found that coho, cutthroat and yearling steelhead rearing densities decreased linearly as temperatures exceeded 17° C (Frissell,1992). They also found that coho salmon juveniles were absent in waters that reached 21 - 23° C, except where thermal refugia were available. Juvenile salmonids will not persist in streams where temperature stress exceeds some threshold that can be defined by species and duration of high temperatures. In northern California, both Welsh et al. (2001) and Hines and Ambrose (1998) found that coho salmon juveniles did not persist where the floating weekly maximum temperature exceeded 18.3° C for any length of time.
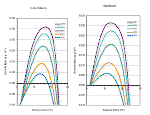 Growth of juveniles is of vital importance for anadromous salmonids that must reach minimum sizes before they can smolt (Weatherly and Gill 1995). Larger size provides a survival benefit through more effective feeding strategies and evasion of predation (Puckett and Dill 1985, Bilton 1982). Growth rates are impaired when water temperature rises above optimum levels. Optimum temperatures for rearing salmonids are generally between 10° C and 16° C, but the actual range for fish in streams varies with food availability and the ability for individuals to obtain that food (i.e. competition, size, and health). At left are temperature growth curves for steelhead and coho.
Growth of juveniles is of vital importance for anadromous salmonids that must reach minimum sizes before they can smolt (Weatherly and Gill 1995). Larger size provides a survival benefit through more effective feeding strategies and evasion of predation (Puckett and Dill 1985, Bilton 1982). Growth rates are impaired when water temperature rises above optimum levels. Optimum temperatures for rearing salmonids are generally between 10° C and 16° C, but the actual range for fish in streams varies with food availability and the ability for individuals to obtain that food (i.e. competition, size, and health). At left are temperature growth curves for steelhead and coho.
| Temperature Tolerance of Coho Salmon Juveniles | |
| UILT: | 26° C (Brett, 1952) |
| CTM : | 24.4° C (McGeer et al.,1991) |
| Growth Stops | 20.3° C (Bell 1973) 19.1° C (Armour, 1991) 18 C° (Stein et al 1972) |
| Optimum Growth | 12-14° C (Brett, 1952) 10-15.6° C (Armour, 1991) 9-13° C (Stein et al 1972) |
| Growth Occurs |
5-17° C (Brungs and Jones 1977) |
UILT = The upper incipient lethal temperature CTM = The critical thermal maxima
Corresponding temperature values on the centigrade and Fahrenheit scales.
Degrees Fahrenheit = (Degrees centigrade x 9/5 ) + 32
| Degrees C | 0 ° (C) | 5 ° (C) | 10° (C) | 15 ° (C) | 20 ° (C) | 25° (C) |
| Degrees F | 32 ° (F) | 41° (F) | 50° (F) | 59° (F) | 68° (F) | 77° (F) |
Adults
McCullough (1999) found that adult chinook salmon and steelhead die at temperatures of 21-22° C in the Columbia River, which demonstrates that adult salmon may have less tolerance for elevated temperatures than juveniles of the same species. Upstream migration ceased at temperatures over 20° C. Bell (1986) defined temperatures suitable for coho salmon adult migration as 7.2-15.6° C. The National Marine Fisheries Service (1996) characterized properly functioning conditions for adult Pacific salmon as between 10-13.9° C and temperatures from 13.9 to 15.5° C as "at risk." McCullough (1999) noted that egg size and development was substantially altered when adults were exposed to temperatures over 17.5° C.
Adult salmon, steelhead and coastal cutthroat trout returning to short coastal streams are unlikely to encounter adverse water temperatures because of moderating influences of the coastal climate and the season of return but even short duration fall temperature peaks could negatively impact adults (Hicks, 2000). Temperatures in longer rivers, such as the Klamath and Eel Rivers, may present substantial problems for late summer and early fall run fish.
Effects of Disease and Competition
Substantial research demonstrates that many fish diseases become more virulent at temperatures over 15.6º C (McCullough, 1999). (Some diseases, like infectious hematopoietic necrosis (IHN), however, are triggered by low water temperatures.) Fish disease organisms are always present in the water, but as young coho or other salmonid species become stressed by higher temperatures their resistance drops. "Also, diseased fish probably are more susceptible to predation and less able to perform essential functions, such as feeding, swimming, and defending territories" (McCullough, 1999). Hatchery reared salmonids are more susceptible to disease than wild fish because of high fish rearing densities.
While salmonids may survive warm water temperatures in laboratory conditions, competition with warm water tolerant species in the wild may make survival problematic. Brown and Moyle (1990) found that salmonids were the most abundant species in cooler stream segments of the Eel River, but warm water fishes dominated in reaches with higher temperature. The northern pike minnow, an introduced warm water adapted species, compete more successfully with young salmon and steelhead as stream temperatures warm and may also prey upon them. Reeves et al. (1987) found steelhead to be dominant in steelhead/shiner interactions when water temperatures ranged from 12-15º C, but that shiners were dominant when water temperatures were 19ºC-22º C. This study revealed how competitive interactions can reduce the ability of salmonids to maintain feeding stations and grow in streams with warmer temperatures (McCullough, 1999).
Factors That Influence Water Temperature
 California's Forest Practice Rules recognize the need to maintain riparian trees in order to provide direct shade and prevent elevation of water temperature. Bartholow (1989), however, found that air temperature above the stream surface was the greatest factor in increasing water temperatures followed in importance by relative humidity and shade, respectively (see graph below). Bartholow's (1989) work was based on field data from hundreds of locations throughout the West used to develop the SNTEMP stream temperature model. While many previous works considered direct solar radiation to be the dominant mechanism for warming streams (Brown, 1980 as cited in Spence et al., 1998), most of the recent scientific literature considers air temperature over the stream to be the most influential factor (see quote from Essig, 1998 ). Poole and Berman (1999) also recognize the relationship between increasing air flow over the stream and water temperature elevation (see quote). Brosofske et al (1997) found that upslope soil temperatures were also a predictor of water temperature.
California's Forest Practice Rules recognize the need to maintain riparian trees in order to provide direct shade and prevent elevation of water temperature. Bartholow (1989), however, found that air temperature above the stream surface was the greatest factor in increasing water temperatures followed in importance by relative humidity and shade, respectively (see graph below). Bartholow's (1989) work was based on field data from hundreds of locations throughout the West used to develop the SNTEMP stream temperature model. While many previous works considered direct solar radiation to be the dominant mechanism for warming streams (Brown, 1980 as cited in Spence et al., 1998), most of the recent scientific literature considers air temperature over the stream to be the most influential factor (see quote from Essig, 1998 ). Poole and Berman (1999) also recognize the relationship between increasing air flow over the stream and water temperature elevation (see quote). Brosofske et al (1997) found that upslope soil temperatures were also a predictor of water temperature.
Welsh et al. (unpublished) found a relationship between the age and abundance of riparian forests and the summer water temperature regime in the Mattole watershed. Temperatures in Mattole River tributaries and reaches were often above thermal limits for coho salmon and sensitive amphibian species, such as the tailed frog and southern torrent salamander (Welsh, unpublished data; see Amphibian page). These studies clearly demonstrate a need for adequate riparian buffers to protect riparian conditions in areas of managed forests.
Maintaining natural functions and processes in the riparian zone is essential for maintaining native biodiversity (Naiman et al., 1993; Ward, 1998). In the Mad River area of northern California, Ledwith (1996) indicated that a 30 meter minimum buffer width on each streamside would be required to ameliorate upslope influences and maintain uniform humidity and air temperatures within the riparian area. Brosofske et al (1997) recommended a minimum 45 meter buffer width for western Washington; an area with less extreme temperatures than northern California. In either case, this suggests that more extensive riparian buffer strips are necessary if optimal water temperatures are to be maintained for salmonids. Spence et al. (1996) recommend that no commercial timber harvest take place within one site potential tree height of a stream (200 feet on either side) when a stream's temperature is higher than its normal range variability.
Sedimentation of streams may also contribute to elevated water temperatures. Sediment can fill pools and cause the width-to-depth ratio of a stream to increase, which can facilitate heat exchange (Poole and Berman, 1999). Hagans et al. (1986) reported that sedimentation caused stream temperatures to increase, as dark-colored fine sediment replaced lighter- colored course gravels. The darker sediment stored more solar radiation. Fine sediment may block exchange between surface waters and intragravel flows, also contributing to warming. Poole and Berman (1999) noted that large wood jams can contribute to stream cooling by forcing more stream flow into shallow ground water, which is called the hyporheic zone. The water drops slightly in temperature before emerging downstream.
Lewis et al. (2000) point out that 1) watersheds that are nearer the coast have cool air temperatures, which makes them less susceptible to stream warming, 2) water temperatures have a tendency to increase with increasing distance from the watershed divide and with increasing drainage area, 3) headwater stream temperatures are close to ground water temperatures, and 4) 70 km and further from their source, most streams in northwestern California were too wide to be affected by canopy cover.
References
Armour, C.L. 1991. Guidance for evaluating and recommending temperature regimes to protect fish. U.S. Fish and Wildlife Service. Fort Collins. Biological Report 90(22). 13 p.
Bartholow, J.M. 1989 . Stream temperature investigations: field and analytic methods. Instream flow information paper no. 13. Biological Report 89(17). U.S. Fish and Wildlife Service, Fort Collins, Co.
Bell, M.C. 1973. Fisheries handbook of engineering requirements and biological criteria. US Army Corps of Engineers. Fish Passage Development and Evaluation Program, North Pacific Division, Portland, Oregon. Contract DACW57-68-0086.
Brett, J.R. 1952. Temperature tolerance in young Pacific salmon, genus Oncorhynchus. J. Fish. Res. Board of Canada. 9(6):265-323. http://www.humboldt.edu/~fsp/tim/1952article.html
Brosofske, K. B., J. Chen, R. J. Naiman, and J. F. Franklin. 1997. Harvesting effects on microclimatic gradients from small streams to uplands in western Washington. Ecological Applications 7(4):1188-1200.
Brown, L. and P.B. Moyle. 1990. Eel River Survey: Final Report. Performed under contract to Calif. Dept. of Fish and Game. Dept. of Wildlife and Fisheries Biology, University of California at Davis.
Brungs, W.A. and B.R. Jones. 1977. Temperature criteria for freshwater fish: protocol and procedures. EPA-600-3-77-061. Environmental Research Laboratory-Duluth, Office of Research and Development, US Environmental Protection Agency. 136 p.
Essig, D. 1998. The Dilemma of Applying Uniform Temperature Criteria in a Diverse Environment: An Issue Analysis. Idaho Division of Environmental Quality Water Quality Assessment and Standards Bureau, Boise, ID. 34p.
Frissell, C.A. 1992. Cumulative effects of land use on salmonid habitat on southwest Oregon streams. Ph.D. thesis, Oregon State University, Corvalis, OR.
Hagans, D.K., W.E. Weaver, and M.A. Madej. 1986. Long term on-site and off-site effects of logging and erosion in the Redwood Creek Basin in Northern California. Technical Bulletin No. 460. National Council of Air and Streams, New York, New York.
Hicks, M. 2000. Preliminary Review Draft Discussion Paper Evaluating Standards for Protecting Aquatic Life In Washington’s Surface Water Quality Standards Temperature Criteria. Washington State Department of Ecology, Water Quality Program, Watershed Management Section. Olympia, Washington.
Hines, D.H. and J.M. Ambrose. 1998. Evaluation of Stream Temperature Thresholds Based on Coho Salmon (Oncorhynchus kisutch) Presence and Absence in Managed Forest Lands in Coastal Mendocino County, California. Georgia Pacific Corporation, Ft. Bragg, CA. 14 p plus Appendices.
Ledwith, T. 1996. The effects of buffer strip width on air temperature and relative humidity in a stream riparian zone. Watershed Council Networker, Summer, 1996: 6-7. http://watershed.org/news/sum_96/buffer.html
Lewis, T., D.et al. 2000. Executive Summary: Regional Assessment of Stream Temperatures Across Northern California and Their Relationship to Various Landscape-Level and Site-Specific Attributes. Forest Science Project. Humboldt State University Foundation, Arcata, CA. 14 p.
McCullough, D. 1999 . A Review and Synthesis of Effects of Alterations to the Water Temperature Regime on Freshwater Life Stages of Salmonids, with Special Reference to Chinook Salmon. Columbia Intertribal Fisheries Commission, Portland, OR. Prepared for the U.S. Environmental Protection Agency Region 10. Published as EPA 910-R-99-010.
McGeer, J.C., L. Baranyi, and G.K. Iwama. 1991. Physiological responses to challenge tests in six stocks of coho salmon (Oncorhynchus kisutch). Can. J. Fish. Aquat. Sci. 48:1761-1771.
Naiman, R. J., H. Decamps, and M. Pollock. 1993. The role of riparian corridors in maintaining regional biodiversity. Ecological Applications 3(2): 209-212.
National Marine Fisheries Service. 1996 . Coastal Salmon Conservation: Working Guidance for Comprehensive Salmon Restoration Initiatives on the Pacific Coast. NMFS, Northwest Region, Seattle, WA. 6 p.
North Coast Regional Water Quality Control Board. 1996. Water quality control plan -North Coast Region.
Poole, G.C., and C.H. Berman. 2000. Pathways of Human Influence on Water Temperature Dynamics in Stream Channels. U.S. Environmental Protection Agency, Region 10. Seattle, WA. 20 p.
Puckett, K.J. and L.M. Dill. 1985. The energetics of feeding territorality in juvenile coho salmon (Oncorhynchus kisutch). Behavior 42:97-111.
Reeves, G.H., F.H. Everest, and J.D. Hall. 1987. Interaction between redside shiner (Richarsonius balteatus) and the steelhead trout (Salmo gairdneri) in western Oregon: the influence of water temperature. Can. J. Fisheries and Aquatic Sciences 44:1603-1613.
Reiser, D. and T. Bjornn. 1979. Habitat Requirements of Anadromous Salmonids. In the series Influence of Forest and Range Management on Anadromous Fish Habitat in Western North America. U.S. Forest Service Forest and Range Experiment Station, Portland, OR. Gen. Tech. Rep. PNW-96. 54 p.
Spence, B.C, G.A. Lomnicky, R.M. Hughes and R.P. Novitski. 1996. An ecosystem approach to salmonid conservation. TR-4501-96-6057. ManTech Corp, Corvalis, OR. Funded by National Marine Fisheries Service, U.S. Fish and Wildlife Service and the U.S. Environmental Protection Agency. Available from NMFS, Portland, OR. http://www.nwr.noaa.gov/1habcon/habweb/ManTech/front.htm#TOC
Stein, R.A., P.E. Reimers, and J.D. Hall. 1972. Social interactions between juvenile coho (Oncorhynchus kisutch) and fall chinook (O. tshawytscha) in Sixes River, Oregon. Journal of Fisheries Resources Board of Canada. 29(12): 1737-1748.
Sullivan, K., D.J. Martin, R.D. Cardwell, J.E. Toll, and S. Duke. 2000. An analysis of the effects of temperature on salmonids of the Pacific Northwest with implications for selecting temperature criteria. Sustainable Ecosystems Institute. Portland, OR. 192 pp. [1.5Mb] http://www.sei.org/downloads/reports/salmon2000.pdf
Ward, J. V. 1998. Riverine landscapes: Biodiversity patterns, disturbance regimes, and aquatic conservation. Biological Conservation Vol. 83, No. 3: 269-278.
Washington State Department of Ecology. 1996 . Water Quality Standards for Aquatic Life. Wash. Dept. of Ecology, Olympia, WA.
Washington State Department of Ecology. 1998. Preliminary Draft Evaluation Standards for Protection of Aquatic Life in Washington Surface Waters. Wash. Dept. of Ecology, Olympia, WA. January 1998.
Weatherly, A.H. and H.S. Gill. 1995. Growth. Pages 101-158 In: [ed] C. Groot, L. Margolis, and W.C. Clarke. Physiological ecology of Pacific Salmon. UBC Press, Vancouver, B.C. Canada.
Welsh, Hartwell. Personal Communication. USFS Redwood Sciences Lab, Arcata, CA.
Welsh, H.H., G.R. Hodgson, M.F. Roche, B.C. Harvey. (2001). Distribution of Juvenile Coho Salmon (Oncorhynchus kisutch) in Relation to Water Temperature in Tributaries of a Northern California Watershed Determining Management Thresholds for an Impaired Cold-water Adapted Fauna. In review for publication in the North American Journal of Fisheries Management. 21:464-470, 2001. http://www.rsl.psw.fs.fed.us/projects/wild/welsh/welsh5.pdf